1. Introduction to transport and excretion
2. Transport in plants
3. Osmosis
4. Transpiration
5. Transport of food
6. Blood, components of blood
7. Blood vessels
8. The heart, Heartbeat and pulse
9. Removal of waste, Dialysis.
MAIN TEACHINGOral and explanation with some written work.
1. Transport in plants
Watch this video now...
2. OsmosisWatch this video now....
6. Blood vessels
7. The heart, Heartbeat and pulse
8. Removal of waste, Dialysis.
STUDENTS TAKE AWAYComplete all exercise questions from given link of chapter.ASSIGNMENTComplete the following questions-Q.1. Define the RBC (red blood cell).Q.2. What is platelet?Q.3. What is the function of arteries?Q.4. What is the function of veins?Q.5. What is a heart?Q.6. How many types of blood vessels are there? Name them.Q.7. Name the four chambers of the heart.Q.8. Where is the human heart located?
R/14 16/11/2021 to 24/11/2021 PHYSICSTOPIC: Introduction, Hot and Cold, Measuring temperature, Reading a thermometer, Laboratory thermometer, Clinical Thermometer, Transfer of heat, Kinds of clothes we wear in summer and winter.
EXPLAINED1. Introduction:- Hot and Cold, Measuring temperature.2. Reading a thermometer-Fahrenheit and degree Celsius scale.3. Laboratory thermometer and Clinical Thermometer4. Transfer of Heat:- Conduction, Convection and Radiation 5. Kinds of clothes we wear in summer and winter.
2. Transport in plants
3. Osmosis
4. Transpiration
5. Transport of food
6. Blood, components of blood
7. Blood vessels
8. The heart, Heartbeat and pulse
9. Removal of waste, Dialysis.
1. Transport in plants
Watch this video now...
STUDENTS TAKE AWAYComplete all exercises from the given link below.ASSIGNMENTComplete All Questions-1. State similarities and differences between the laboratory thermometer and the clinical thermometer.2. Give two examples each of conductors and insulators of heat.3. Fill in the blanks:(a) The hotness of an object is determined by its __________.(b) Temperature of boiling water cannot be measured by a _____________ thermometer.
(c) Temperature is measured in degree ______________.
(d) No medium is required for transfer of heat by the process of __________.
(e) A cold steel spoon is dipped in a cup of hot milk. It transfers heat to its other end by the process of ______________.
(f ) Clothes of ______________ colours absorb heat better than clothes of light colours.
(c) Temperature is measured in degree ______________.
(d) No medium is required for transfer of heat by the process of __________.
(e) A cold steel spoon is dipped in a cup of hot milk. It transfers heat to its other end by the process of ______________.
(f ) Clothes of ______________ colours absorb heat better than clothes of light colours.
4. Match the following:(i) Land breeze blows during (a) summer
5. Discuss why wearing more layers of clothing during winter keeps us warmer than wearing just one thick piece of clothing.
Choose the correct Option-6. One litre of water at 30°C is mixed with one litre of water at 50°C. The temperature of the mixture will be
7. An iron ball at 40°C is dropped in a mug containing water at 40°C. The heat will
(a) Flow from iron ball to water.
(b) Not flow from iron ball to water or from water to iron ball.
(c) Flow from water to iron ball.
(d) Increase the temperature of both.
8. A wooden spoon is dipped in a cup of ice cream. Its other end
(a) becomes cold by the process of conduction.
(b) becomes cold by the process of convection.
(c) becomes cold by the process of radiation.
(d) does not become cold.
9. Stainless steel pans are usually provided with copper bottoms. The reason for this could be that
(a) Copper bottom makes the pan more durable.
(b) Such pans appear colourful.
(c) Copper is a better conductor of heat than the stainless steel.(d) Copper is easier to clean than the stainless steel.
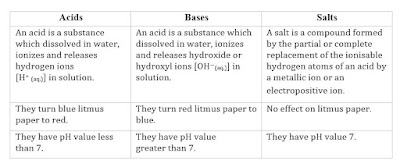
R/13 31/10/2021 to 15/11/2021 LINKSTOPIC: Introduction, Acids and Bases, Natural indicators and Synthetic indicators, Properties of acids, bases and salts, Neutralization, Neutralization in our daily life.
Choose the correct Option-
(a) Flow from iron ball to water.
(b) Not flow from iron ball to water or from water to iron ball.
(c) Flow from water to iron ball.
(d) Increase the temperature of both.
8. A wooden spoon is dipped in a cup of ice cream. Its other end
(a) becomes cold by the process of conduction.
(b) becomes cold by the process of convection.
(c) becomes cold by the process of radiation.
(d) does not become cold.
9. Stainless steel pans are usually provided with copper bottoms. The reason for this could be that
(a) Copper bottom makes the pan more durable.
(b) Such pans appear colourful.
(c) Copper is a better conductor of heat than the stainless steel.
1. Introduction:-Acids, Bases and Salts
MAIN TEACHINGOral and explanation with some written work
2. Indicators and their types:-Synthetic and natural indicators.3. Properties of acids and bases.
4. Neutralization and their application in our daily life.STUDENTS TAKE AWAYComplete all exercises from the link given below.
ASSIGNMENT
1. Look at the given reaction.
Hydrochloric acid + Sodium hydroxide (base) → Sodium chloride (salt) + Water
Sodium chloride formed in this reaction remains in solution form.
Can we get solid sodium chloride from this solution? Suggest a method (if any).
2. State whether the following statements are true or false.
Correct the false statements.
(a) All substances are either acidic or basic.
(b) A compound if acidic will turn all indicators red.
(c) Lime water turns red litmus blue.
(d) Common salt dissolved in water turns blue litmus red.
(e) Phenolphthalein is a natural indicator.
(f) Calamine can be used to treat ant’s sting.
(g) Lemon water is basic in nature.
3. Paheli is suffering from indigestion due to acidity. Is it advisable to give her orange juice in this situation and why?
Short Answer Type Questions
1.Look at Figure 5.1 which shows solutions taken in test tubes A,B,C and D. What colour is expected when a piece of red litmus paper is dropped in each test tube?Nature of the solutions is given in the table for your help.2. While playing in a park, a child was stung by a wasp. Some elders suggested applying paste of baking soda and others lemon juice as remedy. Which remedy do you think is appropriate and why?
3. Form a sentence using the following words – baking soda, antbite, moist, effect, neutralised, rubbing.
4. Match the substances in Column I with those in Column II.
5. Fill the blanks in the following sentences.
(a) Lemon juice and vinegar taste ___________ because they contain ___________.
(b) Turmeric and litmus are _________ acid-base indicators.
(c) Phenolphthalein gives _________ colour with lime water.
(d) When an acidic solution is mixed with a basic solution, they _________ each other forming _________ and water.
Long Answer Type Questions 1. Boojho, Paheli and their friend Golu were provided with a test tube each containing China rose solution which was pink in colour. Boojho added two drops of solution ‘A’ in his test tube and got dark pink colour. Paheli added 2 drops of solution ‘B’ to her test tube and got green colour. Golu added 2 drops of solution ‘C’ but could not get any change in colour. Suggest the possible cause for the variation in their results.
2. Fill in the cross word given as Figure 5.2 with the help of the clues provided.
Across(2) The solution which does not change the color of either red or blue litmus.
4.You are provided with four test tubes containing sugar solution, baking soda solution, tamarind solution, salt solution. Write down an activity to find the nature (acidic/basic/neutral) of each solution.
5. You are provided with three test tubes A, B and C as shown in Figure 5.3
with different liquids. What will you observe when you put
(a) a piece of blue litmus paper in each test tube.
(b) a piece of red litmus paperin each test tube.
(c) a few drops of phenolphthalein solution to each test tube. 6. Paheli observed that most of the fish in the pond of her village were gradually dying. She also observed that the waste of a factory in their village is flowing into the pond which probably caused the fish to die.
7. Explain two neutralization reactions related to daily life.
D. Complete the following.
2. Ammonium hydroxide is an alkali.
3. An acid is neutralised by a base.
4. An antacid generally contains a base.
5.
Indicator
Colour
Acid
Base
Litmus
Red
Blue
Phenolphthalein
Colourless
Red
Turmeric juice
Yellow
Reddish-brown
Red cabbage juice
Red
Green
China rose juice
Red
Green
E. Choose the correct option.
2. ANSWER: (d) sodium hydroxide
3. ANSWER: (b) alkaline
4. ANSWER: (d) salt and water
F. Match the columns A and B.
A
B
(a) Hydrochloric acid
(iv) As bathroom acid
(b) Ascorbic acid
(v) Vitamin C
(c) Sulphuric acid
(i) In storage batteries
(d) Lactic acid
(ii) Found in Yoghurt
(e) Acetic acid
(iii) In making vinegar
2. ANSWER:
A
B
(a) Sodium iodate
(v) A supplement to common salt
(b) Calcium sulphate
(iii) Present in plaster of Paris
(c) Bleaching powder
(iv) A disinfectant
(d) Ammonium sulphate
(ii) Used as a fertiliser
(e) Sodium benzoate
(i) A food preservative
G. Tick the correct box.
ANSWER:
1. Yes. Most salts are neutral.
2. Yes. Soluble bases are called alkalis.
3. No. Calcium chloride, on heating, loses water of hydration and may undergo
dissociation at very high temperatures to form calcium metal and chlorine gas.
4. Yes. Lemon juice will give carbon dioxide with marble also.
5. Yes. Sulphur when burnt in air produces acetic acid.
1. Look at the given reaction.
Hydrochloric acid + Sodium hydroxide (base) → Sodium chloride (salt) + Water
Sodium chloride formed in this reaction remains in solution form.
Can we get solid sodium chloride from this solution? Suggest a method (if any).
2. State whether the following statements are true or false.
Correct the false statements.
(a) All substances are either acidic or basic.
(b) A compound if acidic will turn all indicators red.
(c) Lime water turns red litmus blue.
(d) Common salt dissolved in water turns blue litmus red.
(e) Phenolphthalein is a natural indicator.
(f) Calamine can be used to treat ant’s sting.
(g) Lemon water is basic in nature.
3. Paheli is suffering from indigestion due to acidity. Is it advisable to give her orange juice in this situation and why?
Short Answer Type Questions
1.Look at Figure 5.1 which shows solutions taken in test tubes A,B,C and D. What colour is expected when a piece of red litmus paper is dropped in each test tube?Nature of the solutions is given in the table for your help.
5. Fill the blanks in the following sentences.
(a) Lemon juice and vinegar taste ___________ because they contain ___________.
(b) Turmeric and litmus are _________ acid-base indicators.
(c) Phenolphthalein gives _________ colour with lime water.
(d) When an acidic solution is mixed with a basic solution, they _________ each other forming _________ and water.
Long Answer Type Questions
2. Fill in the cross word given as Figure 5.2 with the help of the clues provided.
Across
4.You are provided with four test tubes containing sugar solution, baking soda solution, tamarind solution, salt solution. Write down an activity to find the nature (acidic/basic/neutral) of each solution.
(a) a piece of blue litmus paper in each test tube.
(b) a piece of red litmus paperin each test tube.
(c) a few drops of phenolphthalein solution to each test tube.
2. Ammonium hydroxide is an alkali.
3. An acid is neutralised by a base.
4. An antacid generally contains a base.
5.
|
Indicator |
Colour |
|
|
Acid |
Base |
|
|
Litmus |
Red |
Blue |
|
Phenolphthalein |
Colourless |
Red |
|
Turmeric juice |
Yellow |
Reddish-brown |
|
Red cabbage juice |
Red |
Green |
|
China rose juice |
Red |
Green |
2. ANSWER: (d) sodium hydroxide
3. ANSWER: (b) alkaline
4. ANSWER: (d) salt and water
A | B |
(a) Hydrochloric acid | (iv) As bathroom acid |
(b) Ascorbic acid | (v) Vitamin C |
(c) Sulphuric acid | (i) In storage batteries |
(d) Lactic acid | (ii) Found in Yoghurt |
(e) Acetic acid | (iii) In making vinegar |
2. ANSWER:
A | B |
(a) Sodium iodate | (v) A supplement to common salt |
(b) Calcium sulphate | (iii) Present in plaster of Paris |
(c) Bleaching powder | (iv) A disinfectant |
(d) Ammonium sulphate | (ii) Used as a fertiliser |
(e) Sodium benzoate | (i) A food preservative |
G. Tick the correct box.
1. Yes. Most salts are neutral.
2. Yes. Soluble bases are called alkalis.
3. No. Calcium chloride, on heating, loses water of hydration and may undergo
dissociation at very high temperatures to form calcium metal and chlorine gas.
4. Yes. Lemon juice will give carbon dioxide with marble also.
5. Yes. Sulphur when burnt in air produces acetic acid.
2. Formulae,
3. Valency of different elements,
4. Obtaining of chemical formulae,
5. Chemical Equation.







(b) Why did she feel comfortable after a massage?








(ii) breaks down food to release energy.
(iii) helps the body to get rid of CO2.
(iv) produces water in the cells.
1. Pick the odd-one-out from each of the groups given below on the basis of respiratory organs. Give reason for your answer.
(a) cockroach, grasshopper, snail, ant
(b) lizard, cow, earthworm, snake
(c) crocodile, whale, dolphin, fish
(d) snake, tadpole, crow, goat
2. Which gas present in air is essential for aerobic respiration? What is the role of oxygen during respiration?
3. On an average, an adult human being at rest breathes 15–18 times per minute. The breathing rate, however, may differ under different conditions. Arrange the following activities given in the box in order of increasing breathing rates and give reason for your answer. (Sleeping, cycling, brisk walk, watching T.V.)
4. On a very cold morning, Boojho and Paheli were talking with each other as they walked down to their school. They observed that the air coming out of their mouth looked like smoke. They were amused and wondered how it happened. Help them find the answer.
5. Whenever we feel drowsy or sleepy, we start yawning. Does yawning help us in anyway?
6. Insects and leaves of a plant have pores through which they exchange gases with the atmosphere. Can you write two points of differences between these pores with respect to their position, number and extension into the body?
Long Answer Type Questions
1. Paheli participated in a 400 m race competition held at her school and won the race. When she came home she had mixed feelings of joy and pain as she had cramps in her leg muscles. After a massage she was relieved of the pain. Answer the following questions related to the situation.
(a) What can be the possible reasons for the pain in her legs?
(b) Why did she feel comfortable after a massage?


(ii) movement of diaphragm
(iii) movement of ribs


EXPLAINED1. Introduction
MAIN TEACHING 3. Chemical Reaction and their types
3. Chemical Reaction and their types 5. Decomposition Reaction
5. Decomposition Reaction 6. Displacement Reaction
6. Displacement Reaction 7. Neutralization Reaction
7. Neutralization Reaction STUDENTS TAKE AWAYComplete all Exercise questions from the given link of the chapter in your Science OCB.ASSIGNMENT
STUDENTS TAKE AWAYComplete all Exercise questions from the given link of the chapter in your Science OCB.ASSIGNMENT- Which of the following is a physical change?
- (a) Rusting of iron
- (b) Combustion of magnesium ribbon
- (c) Burning of candle
- (d) Melting of wax
- Which of the following is a chemical change?
- (a) Twinkling of stars
- (b) Cooking of vegetables
- (c) Cutting of fruits
- (d) Boiling of water
- A chemical change may involve –
- (a) change in colour only
- (b) change in temperature only
- (c) evolution of gas only
- (d) any or all of the above
- Which of the following is/are true when milk changes into curd?
(i) Its state is changed from liquid to semi solid.
(ii) It changes colour.
(iii) It changes taste.
(iv) The change cannot be reversed.
Choose the correct option from below :- (a) (i) and (ii) are correct
- (b) (ii) and (iii) are correct
- (c) (i), (iii) and (iv) are correct
- (d) (i) to (iv) are correct
- A man painted his main gate made up of iron, to
(i) prevent it from rusting.
(ii) protect it from sun.
(iii) make it look beautiful.
(iv) make it dust free.
Which of the above statement(s) is/are correct?- (a) (i) and (ii)
- (b) (ii) and (iii)
- (c) only (ii)
- (d) (i) and (iii)
- Iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
- (a) It is more than 7 metres high.
- (b) It weighs about 6000 kg.
- (c) It was built more than 1600 years ago.
- (d) It has not rusted after such a long period.
- Galvanisation is a process used to prevent the rusting of which of the following?
- (a) Iron
- (b) Zinc
- (c) Aluminium
- (d) Copper
- Paheli’s mother made a concentrated sugar syrup by dissolving sugar in hot water. On cooling, crystals of sugar got separated. This indicates a –
- (a) physical change that can be reversed.
- (b) chemical change that can be reversed.
- (c) physical change that cannot be reversed.
- (d) chemical change that cannot be reversed.
- Which of the following statement is incorrect for a chemical reaction?
- (a) Heat may be given out but never absorbed.
- (b) Sound may be produced.
- (c) A colour change may take place.
- (d) A gas may be evolved.
- Two drops of dilute sulphuric acid were added to 1 g of copper sulphate powder and then small amount of hot water was added to dissolve it (step I). On cooling, beautiful blue coloured crystals got separated (step II). Step I and step II are:
- (a) physical and chemical changes respectively.
- (b) chemical and physical changes respectively.
- (c) both physical change.
- (d) both chemical change.



- Which of the following is a physical change?
- (a) Rusting of iron
- (b) Combustion of magnesium ribbon
- (c) Burning of candle
- (d) Melting of wax
- Which of the following is a chemical change?
- (a) Twinkling of stars
- (b) Cooking of vegetables
- (c) Cutting of fruits
- (d) Boiling of water
- A chemical change may involve –
- (a) change in colour only
- (b) change in temperature only
- (c) evolution of gas only
- (d) any or all of the above
- Which of the following is/are true when milk changes into curd?
(i) Its state is changed from liquid to semi solid.
(ii) It changes colour.
(iii) It changes taste.
(iv) The change cannot be reversed.
Choose the correct option from below :- (a) (i) and (ii) are correct
- (b) (ii) and (iii) are correct
- (c) (i), (iii) and (iv) are correct
- (d) (i) to (iv) are correct
- A man painted his main gate made up of iron, to
(i) prevent it from rusting.
(ii) protect it from sun.
(iii) make it look beautiful.
(iv) make it dust free.
Which of the above statement(s) is/are correct?- (a) (i) and (ii)
- (b) (ii) and (iii)
- (c) only (ii)
- (d) (i) and (iii)
- Iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
- (a) It is more than 7 metres high.
- (b) It weighs about 6000 kg.
- (c) It was built more than 1600 years ago.
- (d) It has not rusted after such a long period.
- Galvanisation is a process used to prevent the rusting of which of the following?
- (a) Iron
- (b) Zinc
- (c) Aluminium
- (d) Copper
- Paheli’s mother made a concentrated sugar syrup by dissolving sugar in hot water. On cooling, crystals of sugar got separated. This indicates a –
- (a) physical change that can be reversed.
- (b) chemical change that can be reversed.
- (c) physical change that cannot be reversed.
- (d) chemical change that cannot be reversed.
- Which of the following statement is incorrect for a chemical reaction?
- (a) Heat may be given out but never absorbed.
- (b) Sound may be produced.
- (c) A colour change may take place.
- (d) A gas may be evolved.
- Two drops of dilute sulphuric acid were added to 1 g of copper sulphate powder and then small amount of hot water was added to dissolve it (step I). On cooling, beautiful blue coloured crystals got separated (step II). Step I and step II are:
- (a) physical and chemical changes respectively.
- (b) chemical and physical changes respectively.
- (c) both physical change.
- (d) both chemical change.
Very Short Answer Type Questions
- State whether the following statements are true or false:
- (a) When a candle burns, both physical and chemical changes take place.
- (b) Anaerobic bacteria digest animal waste and produce biogas.
- (c) Ships suffer a lot of damage though they are painted.
- (d) Stretching of rubber band is not a physical change.
- Melting of wax is a change where a solid changes to liquid state. Give one more such change which you observe in your surroundings.
- What kind of change is shown by tearing of paper?
- State whether the following statements are true or false:
- (a) When a candle burns, both physical and chemical changes take place.
- (b) Anaerobic bacteria digest animal waste and produce biogas.
- (c) Ships suffer a lot of damage though they are painted.
- (d) Stretching of rubber band is not a physical change.
- Melting of wax is a change where a solid changes to liquid state. Give one more such change which you observe in your surroundings.
- What kind of change is shown by tearing of paper?
Short Answer Type Questions
- Match the items of Column I with the items of Column II.

- Fill in the blanks in the following statements using the words given in the box.
rusted, colourful, substance, chemical, physical, reversible, iron oxide, object- (a) Making sugar solution is a ____________ change.
- (b) A physical change is generally____________.
- (c) Grinding of wheat grain changes its size. It is a ____________ change.
- (d) Iron benches kept in lawns and gardens get____________.
It is a _________ change because a new _________ is formed.
- Classify the following processes into physical or chemical changes:
- (i) Beating of aluminium metal to make aluminium foil.
- (ii) Digestion of food.
- (iii) Cutting of a log of wood into pieces.
- (iv) Burning of crackers.
- Write word equations for two chemical reactions with the help of materials given in the box.
Air, copper sulphate, iron, vinegar, iron oxide, carbon dioxide, iron sulphate, copper, lime water, water - Explain the following:
- (a) Lime water turns milky on passing carbon dioxide gas into it.
- (b) Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
- Match the items of Column I with the items of Column II.

- Fill in the blanks in the following statements using the words given in the box.
rusted, colourful, substance, chemical, physical, reversible, iron oxide, object- (a) Making sugar solution is a ____________ change.
- (b) A physical change is generally____________.
- (c) Grinding of wheat grain changes its size. It is a ____________ change.
- (d) Iron benches kept in lawns and gardens get____________.
It is a _________ change because a new _________ is formed.
- Classify the following processes into physical or chemical changes:
- (i) Beating of aluminium metal to make aluminium foil.
- (ii) Digestion of food.
- (iii) Cutting of a log of wood into pieces.
- (iv) Burning of crackers.
- Write word equations for two chemical reactions with the help of materials given in the box.
Air, copper sulphate, iron, vinegar, iron oxide, carbon dioxide, iron sulphate, copper, lime water, water - Explain the following:
- (a) Lime water turns milky on passing carbon dioxide gas into it.
- (b) Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Long Answer Type Questions
- Give two examples for each of the following cases:
- (a) Physical changes which are reversible.
- (b) Physical changes which are not reversible.
- (c) Chemical changes.
- Give an example of a chemical reaction for each of the following situations:
- (a) A change in colour is observed.
- (b) A gas is evolved.
- (c) Sound is produced.
- If you leave a piece of iron in the open for a few days, it acquires a film of brownish substance, called rust.
- (a) Do you think rust is different from iron?
- (b) Can you change rust back into iron by some simple method?
- (c) Do you think formation of rust from iron is a chemical change?
- (d) Give two other examples of a similar type of change.
- A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
- (a) What changes do you expect?
- (b) Are these changes chemical in nature?
- (c) Write a word equation for the chemical change, if any.
- Give two examples for each of the following cases:
- (a) Physical changes which are reversible.
- (b) Physical changes which are not reversible.
- (c) Chemical changes.
- Give an example of a chemical reaction for each of the following situations:
- (a) A change in colour is observed.
- (b) A gas is evolved.
- (c) Sound is produced.
- If you leave a piece of iron in the open for a few days, it acquires a film of brownish substance, called rust.
- (a) Do you think rust is different from iron?
- (b) Can you change rust back into iron by some simple method?
- (c) Do you think formation of rust from iron is a chemical change?
- (d) Give two other examples of a similar type of change.
- A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
- (a) What changes do you expect?
- (b) Are these changes chemical in nature?
- (c) Write a word equation for the chemical change, if any.
EXPLAINED1. Introduction
MAIN TEACHING 3. Chemical Reaction and their types
3. Chemical Reaction and their types 4. Combination Reaction
4. Combination Reaction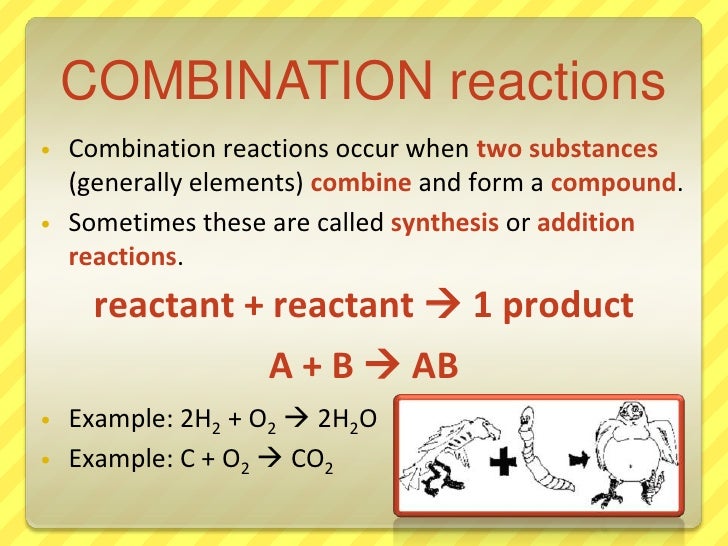 5. Decomposition Reaction
5. Decomposition Reaction 6. Displacement Reaction
6. Displacement Reaction 7. Neutralization Reaction
7. Neutralization Reaction Watch the video now...STUDENTS TAKE AWAYComplete all Exercise questions from the given link of the chapter in your Science OCB.ASSIGNMENT
Watch the video now...STUDENTS TAKE AWAYComplete all Exercise questions from the given link of the chapter in your Science OCB.ASSIGNMENT- Which of the following is a physical change?
- (a) Rusting of iron
- (b) Combustion of magnesium ribbon
- (c) Burning of candle
- (d) Melting of wax
- Which of the following is a chemical change?
- (a) Twinkling of stars
- (b) Cooking of vegetables
- (c) Cutting of fruits
- (d) Boiling of water
- A chemical change may involve –
- (a) change in colour only
- (b) change in temperature only
- (c) evolution of gas only
- (d) any or all of the above
- Which of the following is/are true when milk changes into curd?
(i) Its state is changed from liquid to semi solid.
(ii) It changes colour.
(iii) It changes taste.
(iv) The change cannot be reversed.
Choose the correct option from below :- (a) (i) and (ii) are correct
- (b) (ii) and (iii) are correct
- (c) (i), (iii) and (iv) are correct
- (d) (i) to (iv) are correct
- A man painted his main gate made up of iron, to
(i) prevent it from rusting.
(ii) protect it from sun.
(iii) make it look beautiful.
(iv) make it dust free.
Which of the above statement(s) is/are correct?- (a) (i) and (ii)
- (b) (ii) and (iii)
- (c) only (ii)
- (d) (i) and (iii)
- Iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
- (a) It is more than 7 metres high.
- (b) It weighs about 6000 kg.
- (c) It was built more than 1600 years ago.
- (d) It has not rusted after such a long period.
- Galvanisation is a process used to prevent the rusting of which of the following?
- (a) Iron
- (b) Zinc
- (c) Aluminium
- (d) Copper
- Paheli’s mother made a concentrated sugar syrup by dissolving sugar in hot water. On cooling, crystals of sugar got separated. This indicates a –
- (a) physical change that can be reversed.
- (b) chemical change that can be reversed.
- (c) physical change that cannot be reversed.
- (d) chemical change that cannot be reversed.
- Which of the following statement is incorrect for a chemical reaction?
- (a) Heat may be given out but never absorbed.
- (b) Sound may be produced.
- (c) A colour change may take place.
- (d) A gas may be evolved.
- Two drops of dilute sulphuric acid were added to 1 g of copper sulphate powder and then small amount of hot water was added to dissolve it (step I). On cooling, beautiful blue coloured crystals got separated (step II). Step I and step II are:
- (a) physical and chemical changes respectively.
- (b) chemical and physical changes respectively.
- (c) both physical change.
- (d) both chemical change.




- Which of the following is a physical change?
- (a) Rusting of iron
- (b) Combustion of magnesium ribbon
- (c) Burning of candle
- (d) Melting of wax
- Which of the following is a chemical change?
- (a) Twinkling of stars
- (b) Cooking of vegetables
- (c) Cutting of fruits
- (d) Boiling of water
- A chemical change may involve –
- (a) change in colour only
- (b) change in temperature only
- (c) evolution of gas only
- (d) any or all of the above
- Which of the following is/are true when milk changes into curd?
(i) Its state is changed from liquid to semi solid.
(ii) It changes colour.
(iii) It changes taste.
(iv) The change cannot be reversed.
Choose the correct option from below :- (a) (i) and (ii) are correct
- (b) (ii) and (iii) are correct
- (c) (i), (iii) and (iv) are correct
- (d) (i) to (iv) are correct
- A man painted his main gate made up of iron, to
(i) prevent it from rusting.
(ii) protect it from sun.
(iii) make it look beautiful.
(iv) make it dust free.
Which of the above statement(s) is/are correct?- (a) (i) and (ii)
- (b) (ii) and (iii)
- (c) only (ii)
- (d) (i) and (iii)
- Iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
- (a) It is more than 7 metres high.
- (b) It weighs about 6000 kg.
- (c) It was built more than 1600 years ago.
- (d) It has not rusted after such a long period.
- Galvanisation is a process used to prevent the rusting of which of the following?
- (a) Iron
- (b) Zinc
- (c) Aluminium
- (d) Copper
- Paheli’s mother made a concentrated sugar syrup by dissolving sugar in hot water. On cooling, crystals of sugar got separated. This indicates a –
- (a) physical change that can be reversed.
- (b) chemical change that can be reversed.
- (c) physical change that cannot be reversed.
- (d) chemical change that cannot be reversed.
- Which of the following statement is incorrect for a chemical reaction?
- (a) Heat may be given out but never absorbed.
- (b) Sound may be produced.
- (c) A colour change may take place.
- (d) A gas may be evolved.
- Two drops of dilute sulphuric acid were added to 1 g of copper sulphate powder and then small amount of hot water was added to dissolve it (step I). On cooling, beautiful blue coloured crystals got separated (step II). Step I and step II are:
- (a) physical and chemical changes respectively.
- (b) chemical and physical changes respectively.
- (c) both physical change.
- (d) both chemical change.
Very Short Answer Type Questions
- State whether the following statements are true or false:
- (a) When a candle burns, both physical and chemical changes take place.
- (b) Anaerobic bacteria digest animal waste and produce biogas.
- (c) Ships suffer a lot of damage though they are painted.
- (d) Stretching of rubber band is not a physical change.
- Melting of wax is a change where a solid changes to liquid state. Give one more such change which you observe in your surroundings.
- What kind of change is shown by tearing of paper?
- State whether the following statements are true or false:
- (a) When a candle burns, both physical and chemical changes take place.
- (b) Anaerobic bacteria digest animal waste and produce biogas.
- (c) Ships suffer a lot of damage though they are painted.
- (d) Stretching of rubber band is not a physical change.
- Melting of wax is a change where a solid changes to liquid state. Give one more such change which you observe in your surroundings.
- What kind of change is shown by tearing of paper?
Short Answer Type Questions
- Match the items of Column I with the items of Column II.

- Fill in the blanks in the following statements using the words given in the box.
rusted, colourful, substance, chemical, physical, reversible, iron oxide, object- (a) Making sugar solution is a ____________ change.
- (b) A physical change is generally____________.
- (c) Grinding of wheat grain changes its size. It is a ____________ change.
- (d) Iron benches kept in lawns and gardens get____________.
It is a _________ change because a new _________ is formed.
- Classify the following processes into physical or chemical changes:
- (i) Beating of aluminium metal to make aluminium foil.
- (ii) Digestion of food.
- (iii) Cutting of a log of wood into pieces.
- (iv) Burning of crackers.
- Write word equations for two chemical reactions with the help of materials given in the box.
Air, copper sulphate, iron, vinegar, iron oxide, carbon dioxide, iron sulphate, copper, lime water, water - Explain the following:
- (a) Lime water turns milky on passing carbon dioxide gas into it.
- (b) Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
- Match the items of Column I with the items of Column II.

- Fill in the blanks in the following statements using the words given in the box.
rusted, colourful, substance, chemical, physical, reversible, iron oxide, object- (a) Making sugar solution is a ____________ change.
- (b) A physical change is generally____________.
- (c) Grinding of wheat grain changes its size. It is a ____________ change.
- (d) Iron benches kept in lawns and gardens get____________.
It is a _________ change because a new _________ is formed.
- Classify the following processes into physical or chemical changes:
- (i) Beating of aluminium metal to make aluminium foil.
- (ii) Digestion of food.
- (iii) Cutting of a log of wood into pieces.
- (iv) Burning of crackers.
- Write word equations for two chemical reactions with the help of materials given in the box.
Air, copper sulphate, iron, vinegar, iron oxide, carbon dioxide, iron sulphate, copper, lime water, water - Explain the following:
- (a) Lime water turns milky on passing carbon dioxide gas into it.
- (b) Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Long Answer Type Questions
- Give two examples for each of the following cases:
- (a) Physical changes which are reversible.
- (b) Physical changes which are not reversible.
- (c) Chemical changes.
- Give an example of a chemical reaction for each of the following situations:
- (a) A change in colour is observed.
- (b) A gas is evolved.
- (c) Sound is produced.
- If you leave a piece of iron in the open for a few days, it acquires a film of brownish substance, called rust.
- (a) Do you think rust is different from iron?
- (b) Can you change rust back into iron by some simple method?
- (c) Do you think formation of rust from iron is a chemical change?
- (d) Give two other examples of a similar type of change.
- A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
- (a) What changes do you expect?
- (b) Are these changes chemical in nature?
- (c) Write a word equation for the chemical change, if any.
- Give two examples for each of the following cases:
- (a) Physical changes which are reversible.
- (b) Physical changes which are not reversible.
- (c) Chemical changes.
- Give an example of a chemical reaction for each of the following situations:
- (a) A change in colour is observed.
- (b) A gas is evolved.
- (c) Sound is produced.
- If you leave a piece of iron in the open for a few days, it acquires a film of brownish substance, called rust.
- (a) Do you think rust is different from iron?
- (b) Can you change rust back into iron by some simple method?
- (c) Do you think formation of rust from iron is a chemical change?
- (d) Give two other examples of a similar type of change.
- A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
- (a) What changes do you expect?
- (b) Are these changes chemical in nature?
- (c) Write a word equation for the chemical change, if any.
EXPLAINED..... 5. Heating effect of current,6. Applications of heating effect of current,
MAIN TEACHINGOral and explanation with some written workcontinued...5. Heating effect of current- When an electric current is passed through a conductor; it generates heat due to the hindrance caused by the conductor to the flowing current. The work done in overcoming the hindrance to the current generates heat in that conductor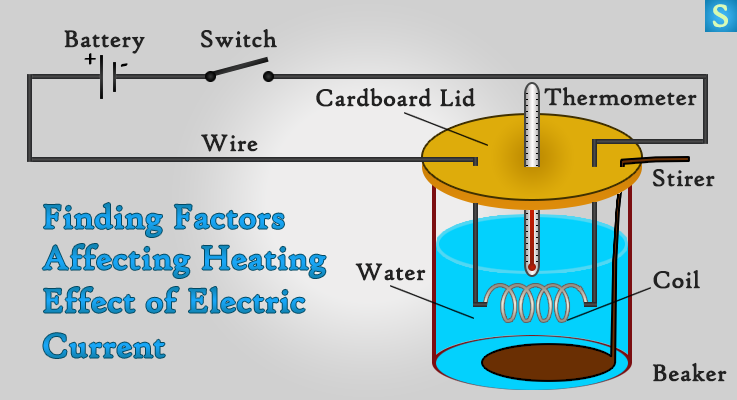 6. Applications of heating effect of current- The heating effect of current is utilised in the working of electrical heating appliances such as electric iron, electric kettle, electric toaster, electric oven, room heaters, water heaters (geysers), etc.7. Electromagnets- Electromagnets are made out of a coil of wire (wire curled in series). This is more effective in producing a magnetic field than just a wire running straight. This effect can be strengthened by winding a wire tightly around a powerful core, made of magnetic material, such as iron.
6. Applications of heating effect of current- The heating effect of current is utilised in the working of electrical heating appliances such as electric iron, electric kettle, electric toaster, electric oven, room heaters, water heaters (geysers), etc.7. Electromagnets- Electromagnets are made out of a coil of wire (wire curled in series). This is more effective in producing a magnetic field than just a wire running straight. This effect can be strengthened by winding a wire tightly around a powerful core, made of magnetic material, such as iron. 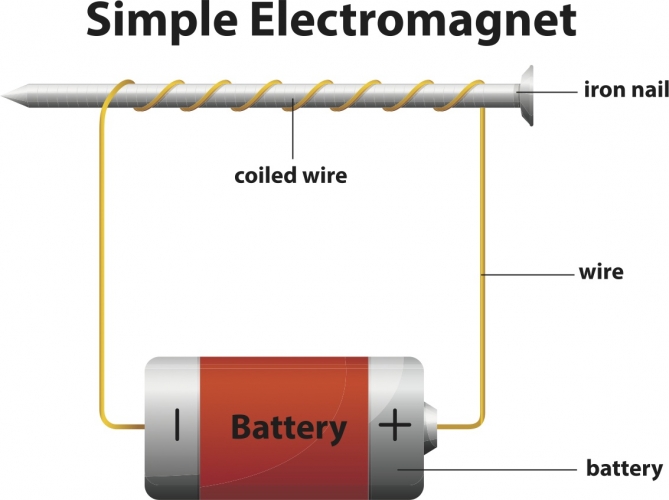 8. Electric bell- An electric bell has an electromagnet that pulls a strip of iron which makes the hammer hit the gong to ring the bell.
8. Electric bell- An electric bell has an electromagnet that pulls a strip of iron which makes the hammer hit the gong to ring the bell. STUDENTS TAKE AWAYComplete all question from the given link of chapter in Science OCB.
STUDENTS TAKE AWAYComplete all question from the given link of chapter in Science OCB.
AssignmentFill in the blanks: (a) The path along which electric current flows is called an______________________(b) Current does not flow in an___________________________ electric circuit. (c) Name the alloy used as the heating element in an electric toaster_________________(d) A fuse wire has a _____________ electric resistance &________________melting point. (e) ________________________discovered that electric currents create magnetic field. (f) The credit for the invention of the electric bulbs goes to__________________________ (g) A combination of two or more cells is called a _________. (h) Full form of MCB is______________________________________________________ (i) Full form of CFL is_______________________________________________________
Name the following : 1. The name of the scientist who first noticed the magnetic effect of current. 2. The switches being used in place of fuses. 3. A diagram made using symbols of electric components. 4. The mark of safety necessary on electrical appliances.
Define : 1. Battery 2. Electromagnet 3. Fuse



EXPLAINED1. Electric current,
MAIN TEACHINGOral and explanation with some written work1. Electric current- Electric Current is the rate of flow of electrons in a conductor. The SI Unit of electric current is the Ampere. 2. Electric circuit- Path for transmitting electric current. An electric circuit includes a device that gives energy to the charged particles constituting the current, such as a battery or a generator; devices that use current, such as lamps, electric motors, or computers; and the connecting wires or transmission lines.
2. Electric circuit- Path for transmitting electric current. An electric circuit includes a device that gives energy to the charged particles constituting the current, such as a battery or a generator; devices that use current, such as lamps, electric motors, or computers; and the connecting wires or transmission lines. 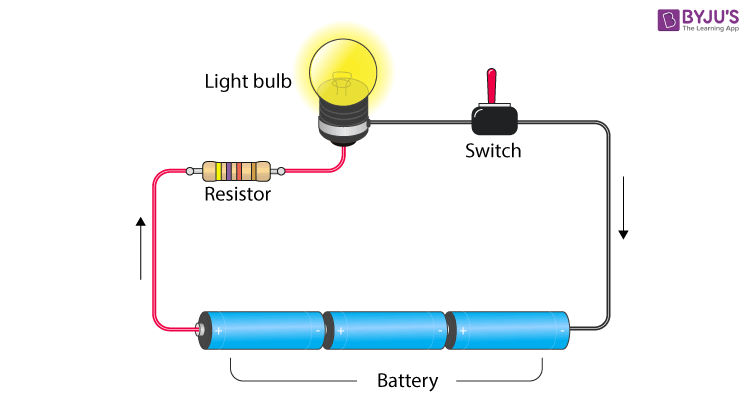 3. Circuit diagram- A circuit diagram is a graphical representation of an electrical circuit.4. Resistance- Electric charge flows easily through some materials than others. The electrical resistance measures how much the flow of this electric charge is restricted within the circuit.
3. Circuit diagram- A circuit diagram is a graphical representation of an electrical circuit.4. Resistance- Electric charge flows easily through some materials than others. The electrical resistance measures how much the flow of this electric charge is restricted within the circuit.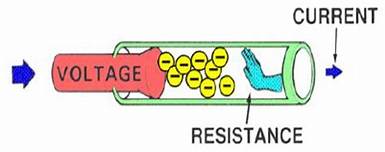 5. Heating effect of current- When an electric current is passed through a conductor; it generates heat due to the hindrance caused by the conductor to the flowing current. The work done in overcoming the hindrance to the current generates heat in that conductor.
5. Heating effect of current- When an electric current is passed through a conductor; it generates heat due to the hindrance caused by the conductor to the flowing current. The work done in overcoming the hindrance to the current generates heat in that conductor.  6. Applications of heating effect of current.7. Electromagnets- Electromagnets are made out of a coil of wire (wire curled in series). This is more effective in producing a magnetic field than just a wire running straight. This effect can be strengthened by winding a wire tightly around a powerful core, made of magnetic material, such as iron.
6. Applications of heating effect of current.7. Electromagnets- Electromagnets are made out of a coil of wire (wire curled in series). This is more effective in producing a magnetic field than just a wire running straight. This effect can be strengthened by winding a wire tightly around a powerful core, made of magnetic material, such as iron.  8. Electric bell- An electric bell has an electromagnet that pulls a strip of iron which makes the hammer hit the gong to ring the bell.
8. Electric bell- An electric bell has an electromagnet that pulls a strip of iron which makes the hammer hit the gong to ring the bell.  STUDENTS TAKE AWAYComplete all question from the given link of chapter in Science OCB.
STUDENTS TAKE AWAYComplete all question from the given link of chapter in Science OCB.
Multiple choice questions
1. When an electric current flows through a copper wire AB as shown in Figure14.1, the wire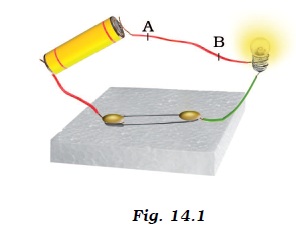
- (a) deflects a magnetic needle placed near it.
- (b) becomes red hot.
- (c) gives electric shock.
- (d) behaves like a fuse.
- Choose the statement which is not correct in the case of an electric fuse.
- (a) Fuses are inserted in electric circuits of all buildings.
- (b) There is a maximum limit on the current which can safely flow through the electric circuits.
- (c) There is a minimum limit on the current which can safely flow in the electric circuits.
- (d) If a proper fuse is inserted in a circuit it will blow off if current exceeds the safe limit.
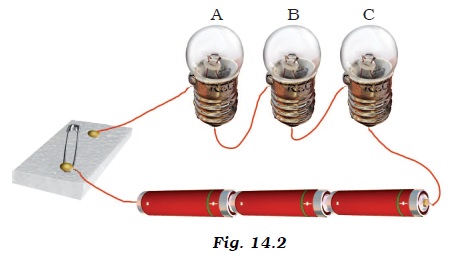
- Three bulbs A, B, C are connected in a circuit as shown in Figure 14.2. When the switch is ‘ON’
- (a) bulb C will glow first.
- (b) bulb B and C will glow simultaneously and bulb A will glow after some time.
- (c) all the bulbs A,B and C will glow at the same time.
- (d) the bulbs will glow in the order A, B and C.
- When a switch is in OFF position,
(i) circuit starting from the positive terminal of the cell stops at the switch.
(ii) circuit is open.
(iii) no current flows through it.
(iv) current flows after some time.
- Choose the combination of correct answer from the following.
- (a) all are correct
- (b) (ii) and (iii) are correct
- (c) only (iv) is correct
- (d) only (i) and (ii) are correct
- Which of the following precautions need not be taken while using electric gadgets/appliances/circuit?
- (a) We should never touch a lighted electric bulb connected to the mains.
- (b) We should never experiment with the electric supply from the mains or a generator or an inverter.
- (c) We should never use just any wire or strip of metal in place of a fuse.
- (d) We should never turn the switch in ON position.





- (a) deflects a magnetic needle placed near it.
- (b) becomes red hot.
- (c) gives electric shock.
- (d) behaves like a fuse.
- Choose the statement which is not correct in the case of an electric fuse.
- (a) Fuses are inserted in electric circuits of all buildings.
- (b) There is a maximum limit on the current which can safely flow through the electric circuits.
- (c) There is a minimum limit on the current which can safely flow in the electric circuits.
- (d) If a proper fuse is inserted in a circuit it will blow off if current exceeds the safe limit.
- Three bulbs A, B, C are connected in a circuit as shown in Figure 14.2. When the switch is ‘ON’
- (a) bulb C will glow first.
- (b) bulb B and C will glow simultaneously and bulb A will glow after some time.
- (c) all the bulbs A,B and C will glow at the same time.
- (d) the bulbs will glow in the order A, B and C.

- When a switch is in OFF position,
(i) circuit starting from the positive terminal of the cell stops at the switch.
(ii) circuit is open.
(iii) no current flows through it.
(iv) current flows after some time. - Choose the combination of correct answer from the following.
- (a) all are correct
- (b) (ii) and (iii) are correct
- (c) only (iv) is correct
- (d) only (i) and (ii) are correct
- Which of the following precautions need not be taken while using electric gadgets/appliances/circuit?
- (a) We should never touch a lighted electric bulb connected to the mains.
- (b) We should never experiment with the electric supply from the mains or a generator or an inverter.
- (c) We should never use just any wire or strip of metal in place of a fuse.
- (d) We should never turn the switch in ON position.
- Which property of a conducting wire is utilized in making electric fuse?
- Name the device used these days in place of electric fuses in electrical circuits.
- Fill in the blanks:
- (i) Our body is a ________________ of electricity.
- (ii) An electric cell produces electricity from the _____________________ in it.
- (iii) In an electric circuit a fuse is a _________ _______ to prevent possible fire.
- (iv) A combination of two or more cells is called a _________.
- Unscramble the following words:
- (i) TBTAYER
- (ii) SFEU
- (iii) HTRCO
- (iv) HICWTS
- Paheli does not have a night lamp in her room. She covered the bulb of her room with a towel in the night to get dim light. Has she taken the right step? Give one reason to justify your answer.
- Why are compact fluorescent lamps (CFLs) preferred over electric bulbs?
- Why is an electric fuse required in all electrical appliances?
- Which property of a conducting wire is utilized in making electric fuse?
- Name the device used these days in place of electric fuses in electrical circuits.
- Fill in the blanks:
- (i) Our body is a ________________ of electricity.
- (ii) An electric cell produces electricity from the _____________________ in it.
- (iii) In an electric circuit a fuse is a _________ _______ to prevent possible fire.
- (iv) A combination of two or more cells is called a _________.
- Unscramble the following words:
- (i) TBTAYER
- (ii) SFEU
- (iii) HTRCO
- (iv) HICWTS
- Paheli does not have a night lamp in her room. She covered the bulb of her room with a towel in the night to get dim light. Has she taken the right step? Give one reason to justify your answer.
- Why are compact fluorescent lamps (CFLs) preferred over electric bulbs?
- Why is an electric fuse required in all electrical appliances?
Short Answer Type Questions
- Can we use the same fuse in a geyser and a television set? Explain.
- Name two electric devices for each where (i) heating effect of current is used and (ii) magnetic effect of current is used.
- Why do we cover plug pin holes which are within the reach of children with cellotape or a plastic cover when not in use?
- Boojho made an electromagnet by winding 50 turns of wire over an iron screw. Paheli also made an electromagnet by winding 100 turns over a similar iron screw. Which electro magnet will attract more pins? Give reason.
- Can we use the same fuse in a geyser and a television set? Explain.
- Name two electric devices for each where (i) heating effect of current is used and (ii) magnetic effect of current is used.
- Why do we cover plug pin holes which are within the reach of children with cellotape or a plastic cover when not in use?
- Boojho made an electromagnet by winding 50 turns of wire over an iron screw. Paheli also made an electromagnet by winding 100 turns over a similar iron screw. Which electro magnet will attract more pins? Give reason.
Long Answer Type Questions
- Your teacher has shown you the following activity.
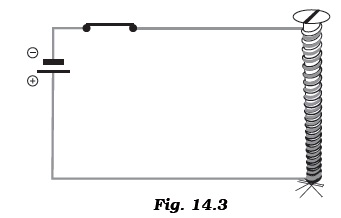 Activity: Teacher has wound a long insulated piece of wire around an iron nail in the form of a coil. Free ends of the wire are connected to a cell through a switch as shown in the Figure 14.3. The current is switched on and some pins are placed near the ends of the nail.
Activity: Teacher has wound a long insulated piece of wire around an iron nail in the form of a coil. Free ends of the wire are connected to a cell through a switch as shown in the Figure 14.3. The current is switched on and some pins are placed near the ends of the nail.
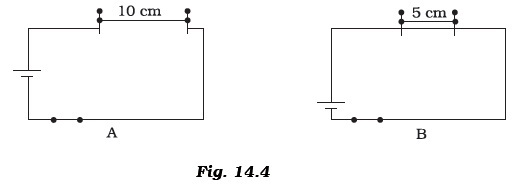
Write down any three questions that come to your mind about this activity. - Paheli took a wire of length 10 cm. Boojho took a wire of 5 cm of the same material and thickness. Both of them connected the wires as shown in the circuit given in Figure 14.4. The current flowing in both the circuits is the same.
(i) Will the heat produced in both the cases be equal? Explain.(ii) Will the heat produced be the same if the wires taken by them are of equal lengths but of different thickness? Explain.
- How does the magnetic effect of electric current help in the working of an electric bell? Explain with the help of a diagram.
- Draw the symbols of the following circuit components.
(i) electric cell (ii) switch in off position (iii) electric bulb (iv) battery
- Your teacher has shown you the following activity.Activity: Teacher has wound a long insulated piece of wire around an iron nail in the form of a coil. Free ends of the wire are connected to a cell through a switch as shown in the Figure 14.3. The current is switched on and some pins are placed near the ends of the nail.

Write down any three questions that come to your mind about this activity. - Paheli took a wire of length 10 cm. Boojho took a wire of 5 cm of the same material and thickness. Both of them connected the wires as shown in the circuit given in Figure 14.4. The current flowing in both the circuits is the same.
(i) Will the heat produced in both the cases be equal? Explain.(ii) Will the heat produced be the same if the wires taken by them are of equal lengths but of different thickness? Explain.
- How does the magnetic effect of electric current help in the working of an electric bell? Explain with the help of a diagram.
- Draw the symbols of the following circuit components.
 |
Multiple Choice Questions
Which of the following cannot be used for measurement of time?
(a) A leaking tap.
(b) Simple pendulum.
(c) Shadow of an object during the day.
(d) Blinking of eyes.
Two clocks A and B are shown in Figure 13.1. Clock A has an hour and a minute hand, whereas clock B has an hour hand, minute hand as well as a second hand. Which of the following statement is correct for these clocks?
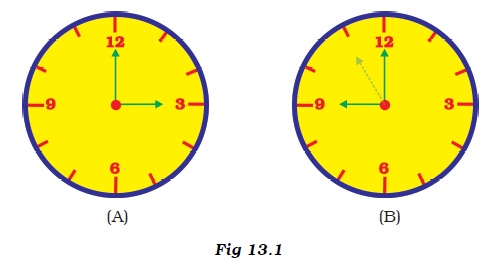
- (a) A time interval of 30 seconds can be measured by clock A.
- (b) A time interval of 30 seconds cannot be measured by clock B.
- (c) Time interval of 5 minutes can be measured by both A and B.
- (d) Time interval of 4 minutes 10 seconds can be measured by clock A.
- Two students were asked to plot a distance-time graph for the motion described by Table A and Table B.The graph given in Figure 13.2 is true for
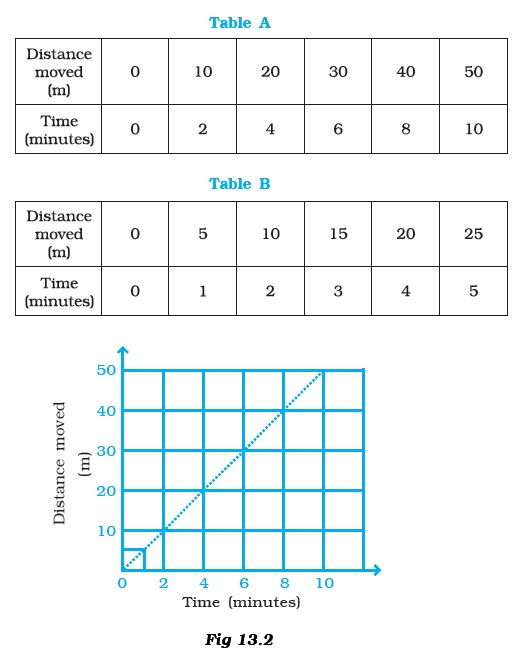
- (a) both A and B.
- (b) A only.
- (c) B only.
- (d) neither A nor B.
- A bus travels 54 km in 90 minutes. The speed of the bus is
- (a) 0.6 m/s
- (b) 10 m/s
- (c) 5.4 m/s
- (d) 3.6 m/s
- If we denote speed by S, distance by D and time by T, the relationship between these quantities is
- (a) S = D × T
- (b) T = S / D
- (c) S = 1 / T * D
- (d) S = T / D
- Observe Figure 13.3.The time period of a simple pendulum is the time taken by it to travel from
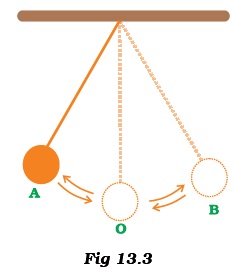
- (a) A to B and back to A.
- (b) O to A, A to B and B to A.
- (c) B to A, A to B and B to O.
- (d) A to B.
- Fig. 13.4 shows an oscillating pendulum.Time taken by the bob to move from A to C is t1 and from C to O is t2. The time period of this simple pendulum is
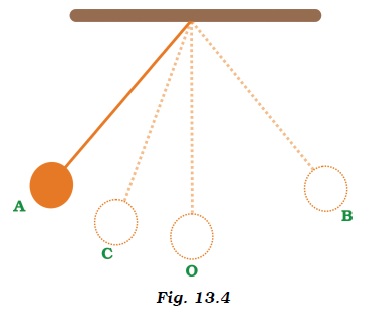
- (a) (t1 + t2 )
- (b) 2 (t1 + t2 )
- (c) 3 (t1 + t2 )
- (d) 4 (t1 + t2 )
- The correct symbol to represent the speed of an object is
- (a) 5 m/s
- (b) 5 mp
- (c) 5 m/s-1
- (d) 5 s/m
- Boojho walks to his school which is at a distance of 3 km from his home in 30 minutes. On reaching he finds that the school is closed and comes back by a bicycle with his friend and reaches home in 20 minutes. His average speed in km/h is
- (a) 8.3
- (b) 7.2
- (c) 5
- (d) 3.6
Very Short Answer Type Questions
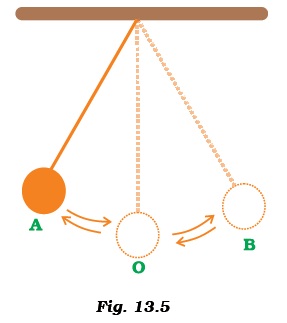
- A simple pendulum is oscillating between two points A and B as shown in Figure 13.5. Is the motion of the bob uniform or non-uniform?
- Paheli and Boojho have to cover different distances to reach their school but they take the same time to reach the school. What can you say about their speed?
- If Boojho covers a certain distance in one hour and Paheli covers the same distance in two hours, who travels in a higher speed?
Short Answer Type Questions
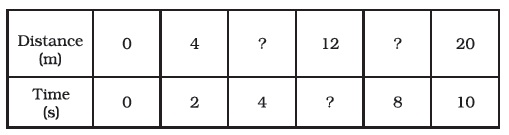
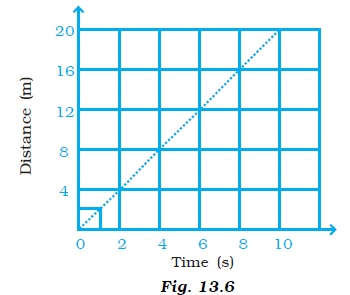
- Complete the data of the table given below with the help of the distance-time graph given in Figure 13.6.
- The average age of children of Class VII is 12 years and 3 months. Express this age in seconds.
- A spaceship travels 36,000 km in one hour. Express its speed in km/s.
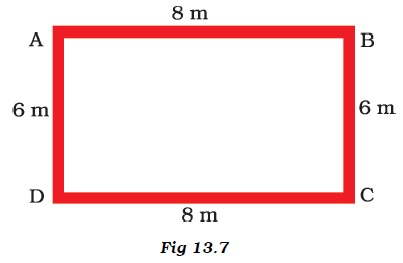
- Starting from A, Paheli moves along a rectangular path ABCD as shown in Figure 13.7. She takes 2 minutes to travel each side. Plot a distance-time graph and explain whether the motion is uniform or non-uniform.
- Plot a distance-time graph of the tip of the second hand of a clock by selecting 4 points on x-axis and y-axis respectively. The circumference of the circle traced by the second hand is 64 cm.
Long Answer Type Questions
- Given below as Figure 13.8 is the distance-time graph of the motion an object.
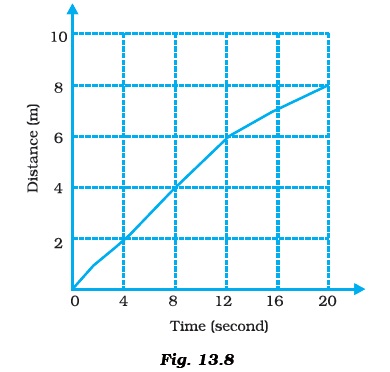
- (i) What will be the position of the object at 20s?
- (ii) What will be the distance travelled by the object in 12s?
- (iii) What is the average speed of the object?
- Distance between Bholu’s and Golu’s house is 9 km. Bholu has to attend Golu’s birthday party at 7 o’clock. He started from his home at 6 o’clock on his bicycle and covered a distance of 6 km in 40 minutes. At that point he met Chintu and he spoke to him for 5 minutes and reached Golu’s birthday party at 7 o’clock. With what speed did he cover the second part of the journey? Calculate his average speed for the entire journey.
- Boojho goes to the football ground to play football. The distance time graph of his journey from his home to the ground is given as Figure 13.9.
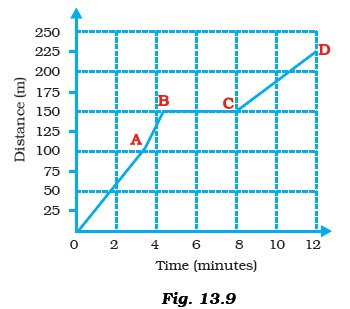
- (a) What does the graph between point B and C indicate about the motion of Boojho?
- (b) Is the motion between 0 to 4 minutes uniform or nonuniform?
- (c) What is his speed between 8 and 12 minutes of his journey
1. Introduction of nutrition and nutrients.
2. Mode of Nutrition: Autotrophic and Heterotrophic.
3. Photosynthesis-food making process
2. Mode of nutrition- autotrophic and heterotrophic.
3. Photosynthesis - food making process.
4. Types of heterotrophic nutrition.
5. Cell-Structural and functional unit of life.
STUDENTS TAKE AWAY
1. How do plants prepare their own food?
2. Why does our body not make food from carbon dioxide, water and minerals like plants do?
3. How does water and minerals, absorbed by the roots reach the leaves?
4. What is special about the leaves that they can synthesize food but other parts of the plant cannot?
*Complete question no. 1-6, from the given chapter links in your OCB notes Ex.books.
Chapter link
ASSIGNMENTS
Extended Extended Learning —
Activities and Projects
- Take a potted plant with broad leaves. Take two strips of black paper and cut out a small square in their centers. Cover a part of two leaves with these papers and secure them with paper clips . Keep the plant in the sunlight for 2–5 days. Observe the difference in the colour of the covered and the uncovered portions on the leaves. Perform iodine test on this leaf. Did the two parts show any difference in results? Now take another leaf. Remove the strip and expose the covered part to the sunlight for 2–3 days and do the iodine test again. Describe your observations.
- to observe and understand how plants are raised and find out how they regulate light, water and carbon dioxide. https://youtu.be/BPJJM_hCFj0
- Try growing a sweet potato just in water. Describe your experiment and observations.
LINKS
Click on the chapter's name to download the chapter in PDF form.
Ch 01 The Language of Chemistry
1. Symbols,
2. Formulae,
3. Valency of different elements,
4. Obtaining of chemical formulae,
5. Chemical Equation.
Oral and explanation with some written work
Complete all assignment questions in OCB.
Q.1. What is chemistry?
Q.2. What is matter?
Q.3. What are the different classifications of matter?
Q.4. What is element?
Q.5. What is symbol?
Q.6. what does a symbol represent?
Q.7. In what way symbol of elopement have been derived?
Q.8. What is valancy ?
Q.9. What is chemical reaction and equation?
Q.10. What are the observations seen in chemical reaction?